This helps in recognizing problem areas beforehand and rectifying them if vital. It might help stay clear of recalls which might be highly-priced and detrimental into the agency’s manufacturer image.
This MLT technique validation protocol is built to determine the method for demonstration which the test specimens to which the test for Microbiological Evaluation of Nonsterile Products: Microbiological Enumeration and Tests for Specified Organisms are applied, usually do not of themselves inhibit the multiplication, underneath the test situations of microorganisms Which might be present.
Head Q.C./Designee – Liable for assessment of your protocol and its summary report for execution of experimental validation examine and arranging methods with the validation program and evaluate of validation results and summary report.
we may well believe that God made the cosmos to be his excellent temple, through which he rested soon after his Imaginative operate. Nonetheless, his Specific revelatory presence did not fill your entire earth nevertheless, since it was his intention that his human vice-regent, whom he put in inside the garden sanctuary, would prolong globally the boundaries of that sanctuary and of God’s existence. Adam, naturally, disobeyed this mandate, to ensure that humanity no longer savored God’s presence inside the very little localized garden.
So as to test a sample to the existence of endotoxins, just one is supposed to incorporate the sample to lysate and that is an enzyme that is certainly derived from the horse shoe crab, precisely derived through the hemolymph cells of the crab.
The presence of Pseudomonas aeruginosa may very well be verified by other appropriate cultural and biochemical tests, if important.
Jovian midnight, during its flyby in 2000, and set novel constraints around the DM-nucleon scattering cross
The principle of Bacterial Endotoxin Test causes it to be essentially more info the most sensitive test that one can use to detect and quantify endotoxins, toxins that are famously recognized for resulting in fever in humans.
To stop contamination, the aseptic technique is the method of minimizing or removing contaminants from coming into the operative industry in medical procedures or drugs.
To determine if the drug is contaminated or its diploma of contamination, and Handle the quality of medication
The MLT tests usually are done on non-sterile pharmaceutical, healthcare and cosmetic products which will range between raw supplies to concluded products.
Head Good quality: Liable for the final acceptance with the MLT strategy protocol and summary report, following completion of qualification summary report shall be checked, reviewed and authorised.
This doc discusses methods for pinpointing pathogenic microorganisms, including germs, fungi, check here and parasites. It describes microscopic evaluation of stained cell preparations and biochemical tests accustomed to discover microbes according to attributes like Gram staining, colony morphology, and hemolysis on blood agar.
Popular mixing mechanisms made use of are disc turbines, vaned discs, and propellers attached to agitator shafts. Spargers may also be discussed for introducing air into the fermentation broth.
 Lark Voorhies Then & Now!
Lark Voorhies Then & Now!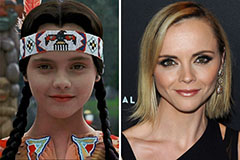 Christina Ricci Then & Now!
Christina Ricci Then & Now!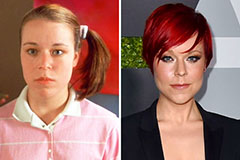 Tina Majorino Then & Now!
Tina Majorino Then & Now! Morgan Fairchild Then & Now!
Morgan Fairchild Then & Now! Rossy de Palma Then & Now!
Rossy de Palma Then & Now!